|
Historical Background
Conceptually, the fuel cell is nothing new. Sir William Grove, widely
regarded as the "Father of the Fuel Cell," experimented with
electrolysis of water as early as 1839. The name itself came about in
1889, when it was coined by inventors Luwdig Mond and Charles Langer.
More information about the early fuel cell experiments is available through
the Fuel Cell Commercialization Group's "What
is a Fuel Cell?" fact sheet.
Internals
 |
|
|
To grasp the concept
of a fuel cell, first visualize a conventional battery. Chemical reactions
within the battery cause electrons to collect on the negative electrode
(anode) of the battery. By connecting the anode to the positive electrode
(cathode), the electrons are free to flow from the negative terminal to
the positive, providing electricity to any devices installed between the
two with a wire.
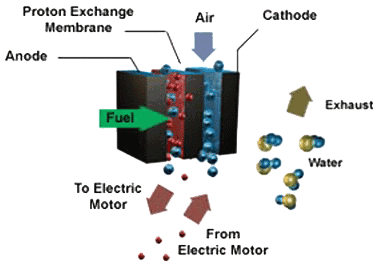
In a fuel cell, the concept is basically the same, except that the chemical
reactions are different. Hydrogen is introduced onto the anode, and a
platinum coating helps to separate it into hydrogen ions and electrons.
In the center of the fuel cell is an elecytolyte membrane which will only
allow the ions to pass through. The electrons leave the fuel cell as electricity.
This reaction is represented by:
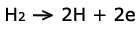
On the other side, oxygen enters the fuel cell on the cathode. A similar
platinum coating allows the oxygen, protons, and leftover electrons to
combine to form water and heat. Thus, the reaction on the cathode is represented
by:
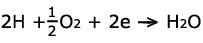
The lifespan of a fuel cell differs from that of a battery as well. Batteries
will eventually deplete their internal supply of chemicals, and the cell
will be useless. Fuel cells (will eventually) run forever, given a fuel
(such as hydrogen) and oxygen. Fuel cells are always ready to run; there
is no warm-up time. The cell will produce electricity the moment a fuel
source and oxygen are
introduced.
Fuel Cell Diagram
 |
|
|
|


![]()
![]()
